How To Find Density With Mass And Diameter
ane.4: Book, Thickness, and Density
- Page ID
- 189531
Learning Objectives
- Calculate surface area, book, density, and thickness and catechumen to various units.
Derived SI Units
We can derive many units from the seven SI base units. For example, we tin can employ the base unit of length to define a unit of volume, and the base units of mass and length to ascertain a unit of density.
Volume
Book is the measure of the amount of space occupied past an object. The standard SI unit of book is defined past the base unit of measurement of length (Figure \(\PageIndex{3}\)). The standard volume is a cubic meter (kthree), a cube with an edge length of exactly one meter. To dispense a cubic meter of h2o, we could build a cubic box with edge lengths of exactly i meter. This box would hold a cubic meter of water or any other substance.
A more than commonly used unit of volume is derived from the decimeter (0.1 m, or 10 cm). A cube with border lengths of exactly 1 decimeter contains a volume of ane cubic decimeter (dm3). A liter (Fifty) is the more mutual name for the cubic decimeter. Ane liter is about 1.06 quarts. A cubic centimeter (cmthree) is the book of a cube with an edge length of exactly i centimeter. The abbreviation cc (for cubic centimeter) is often used by health professionals. A cubic centimeter is besides chosen a milliliter (mL) and is 1/g of a liter.
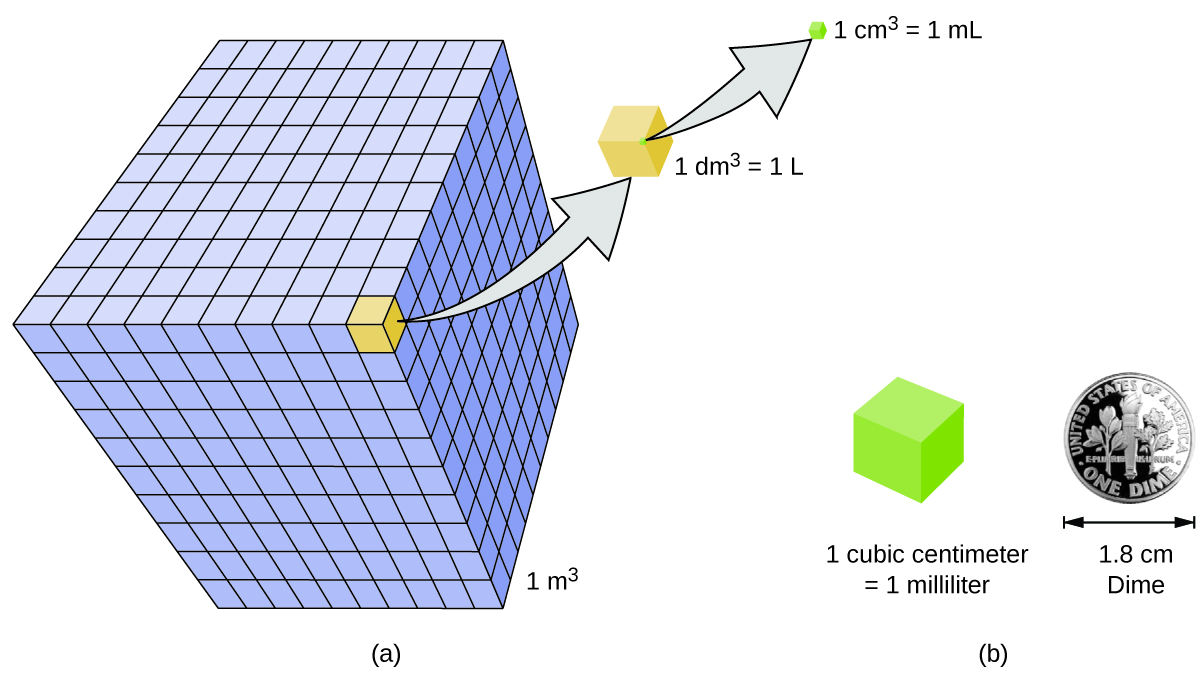 <
< Density
We use the mass and volume of a substance to decide its density. Thus, the units of density are defined by the base units of mass and length.
The density of a substance is the ratio of the mass of a sample of the substance to its volume. The SI unit for density is the kilogram per cubic meter (kg/m3). For many situations, withal, this as an inconvenient unit, and we often apply grams per cubic centimeter (1000/cmthree) for the densities of solids and liquids, and grams per liter (g/L) for gases. Although there are exceptions, nearly liquids and solids accept densities that range from most 0.vii m/cmthree (the density of gasoline) to 19 g/cmiii (the density of gold). The density of air is about i.2 grand/50. Table \(\PageIndex{3}\) shows the densities of some common substances.
| Solids | Liquids | Gases (at 25 °C and 1 atm) |
|---|---|---|
| ice (at 0 °C) 0.92 g/cmiii | water 1.0 g/cmthree | dry air 1.xx 1000/L |
| oak (forest) 0.60–0.90 thou/cmiii | ethanol 0.79 g/cm3 | oxygen i.31 g/L |
| iron vii.9 g/cmthree | acetone 0.79 one thousand/cmiii | nitrogen 1.14 1000/L |
| copper 9.0 g/cm3 | glycerin ane.26 g/cm3 | carbon dioxide 1.80 yard/L |
| atomic number 82 eleven.3 g/cmthree | olive oil 0.92 one thousand/cm3 | helium 0.16 1000/50 |
| silverish 10.5 g/cm3 | gasoline 0.lxx–0.77 thousand/cm3 | neon 0.83 g/L |
| aureate 19.3 g/cm3 | mercury 13.six grand/cm3 | radon nine.1 g/L |
While in that location are many ways to determine the density of an object, perhaps the most straightforward method involves separately finding the mass and book of the object, then dividing the mass of the sample by its volume. In the following case, the mass is found straight by weighing, but the volume is establish indirectly through length measurements.
\[\mathrm{density=\dfrac{mass}{volume}}\]
Example \(\PageIndex{one}\)
Calculation of Density Gold—in bricks, bars, and coins—has been a form of currency for centuries. In club to swindle people into paying for a brick of golden without actually investing in a brick of gold, people accept considered filling the centers of hollow gilt bricks with pb to fool buyers into thinking that the entire brick is gold. It does not work: Lead is a dumbo substance, but its density is not as cracking as that of gilded, 19.3 g/cmthree. What is the density of pb if a cube of lead has an border length of 2.00 cm and a mass of 90.7 g?
Solution
The density of a substance can be calculated by dividing its mass by its volume. The volume of a cube is calculated past cubing the border length.
\[\mathrm{book\: of\: pb\: cube=two.00\: cm\times2.00\: cm\times2.00\: cm=viii.00\: cm^3}\]
\[\mathrm{density=\dfrac{mass}{book}=\dfrac{90.7\: g}{8.00\: cm^3}=\dfrac{11.3\: g}{1.00\: cm^three}=11.3\: thou/cm^iii}\]
Exercise \(\PageIndex{i}\)
- To three decimal places, what is the volume of a cube (cm3) with an edge length of 0.843 cm?
- If the cube in part (a) is copper and has a mass of v.34 g, what is the density of copper to two decimal places?
- Answer a
-
0.599 cm3;
- Reply b
-
8.91 g/cm3
Example \(\PageIndex{two}\): Using Displacement of Water to Make up one's mind Density
This PhET simulation illustrates some other manner to determine density, using displacement of water. Determine the density of the red and xanthous blocks.
Solution
When you open the density simulation and select Same Mass, y'all can choose from several 5.00-kg colored blocks that you tin can drop into a tank containing 100.00 L h2o. The yellow cake floats (it is less dense than water), and the water level rises to 105.00 L. While floating, the xanthous block displaces 5.00 50 water, an amount equal to the weight of the block. The red block sinks (it is more dumbo than water, which has density = 1.00 kg/Fifty), and the water level rises to 101.25 L.
The ruby-red block therefore displaces 1.25 50 water, an amount equal to the volume of the block. The density of the ruddy block is:
\[\mathrm{density=\dfrac{mass}{volume}=\dfrac{5.00\: kg}{ane.25\: Fifty}=four.00\: kg/L}\]
Annotation that since the yellowish block is not completely submerged, you cannot decide its density from this information. Just if you hold the yellow block on the lesser of the tank, the h2o level rises to 110.00 L, which means that information technology now displaces x.00 L water, and its density can be found:
\[\mathrm{density=\dfrac{mass}{volume}=\dfrac{5.00\: kg}{10.00\: L}=0.500\: kg/L}\]
Exercise \(\PageIndex{one}\)
Remove all of the blocks from the h2o and add the green cake to the tank of water, placing it approximately in the heart of the tank. Determine the density of the light-green cake.
- Reply
-
two.00 kg/L
Thickness
The width of an object is also sometimes defined as thickness (T). This generally occurs when the width of the object is significantly smaller than the other dimensions. Measuring thickness requires a tool with great precision such as calipers. However, if you know the area, mass, and density of a substance, then you could calculate the thickness.
Use this formula to calculate thickness: \(\mathrm{thickness=\dfrac{volume}{expanse}}\)
To observe the book, you would rearrange the density formula. \(\mathrm{volume=\dfrac{mass}{density}}\)
Instance \(\PageIndex{ii}\): Calculating Thickness
A piece of aluminum foil has a mass of 0.018g and is 5.0cm on each side. Given the density of aluminum is 2.7g/cc, what is the thickness of aluminum in cm?
Solution
\(\mathrm{thickness=\dfrac{volume}{surface area}}\)
First find the book of the foil.
\[\mathrm{volume=\dfrac{mass}{density}=\dfrac{0.018\: g}{2.7\: k/cc}=0.00667\: cc}\]
Next notice the area of the foil.
\[\mathrm{expanse =5.0\: cm\times5.0\: cm=25\: cm^2}\]
Solve for Thickness
\[\mathrm{thickness=\dfrac{volume}{area}=\dfrac{0.00667\: cc}{25\: cm^2}=0.00027\: cm}\]
Summary
Scientists apply derived units, such equally liters (for volume) and g/cm3 (for density). Thickness is a way to express the width of an object when that dimension is minor.
Key Equations
- \(\mathrm{density=\dfrac{mass}{volume}}\)
- \(\mathrm{thickness=\dfrac{volume}{area}}\)
Glossary
- density
- ratio of mass to book for a substance or object
- liter (Fifty)
- (also, cubic decimeter) unit of measurement of volume; one L = 1,000 cm3
- milliliter (mL)
- 1/ane,000 of a liter; equal to 1 cm3
- second (southward)
- SI unit of time
- SI units (International Organization of Units)
- standards fixed by international understanding in the International System of Units (Le Système International d'Unités)
- thickness
- another mode to limited the width of an object which can be caluculated by taking the volume of the object divided by the area
- volume
- amount of space occupied by an object
Source: https://chem.libretexts.org/Courses/Oregon_Institute_of_Technology/OIT%3A_CHE_101_-_Introduction_to_General_Chemistry/01%3A_Making_Measurements/1.04%3A_Volume_Thickness_and_Density
Posted by: fullercultin.blogspot.com


0 Response to "How To Find Density With Mass And Diameter"
Post a Comment